Viral load

The Snapshot
- Susceptible cattle that are exposed to Pestivirus can face a variety of negative impacts before they recover and become immune.
- Significant complications arise when Pestivirus infection impacts a cow during the pregnancy as the virus infects the calf foetus.
- Without the existence of persistently infected cattle, the spread of Pestivirus would be much easier to contain/manage and perhaps even eradicate.
- Despite the prevalence of Pestivirus in the Australian herd, the impact it has upon cattle, and the cost to the sector there is no standard industry wide disease control messaging.
The Detail
Episode3 (EP3), with the support of Zoetis Australia, have been investigating the impact of Bovine Viral Diarrhoea Virus (BVDV), also known as Pestivirus, on the Australian cattle sector and this piece is the second in a series of articles on Pestivirus that will focus upon:
- What the virus is, how widely it is distributed and how it is spread (Published 14th October)
- The impact of the virus on cattle, types of infected animals, implications for breeding and current control measures
- The economic cost of the virus, impacts to the beef feedlot and dairy sector
- The international response to the virus and the consideration of a control/eradication program for Australia
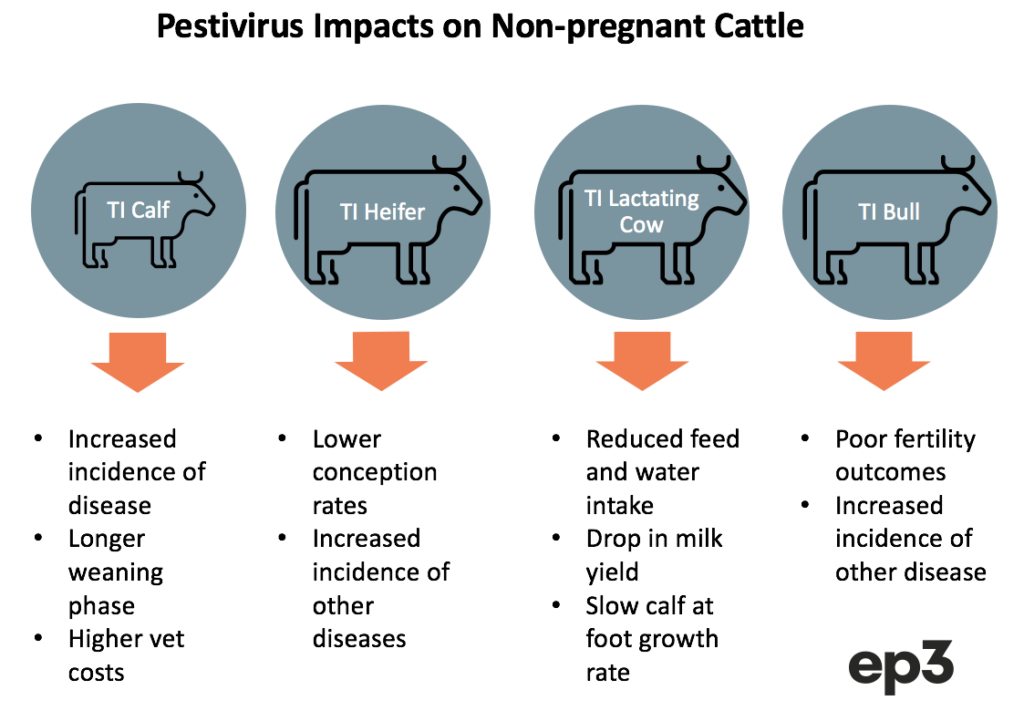
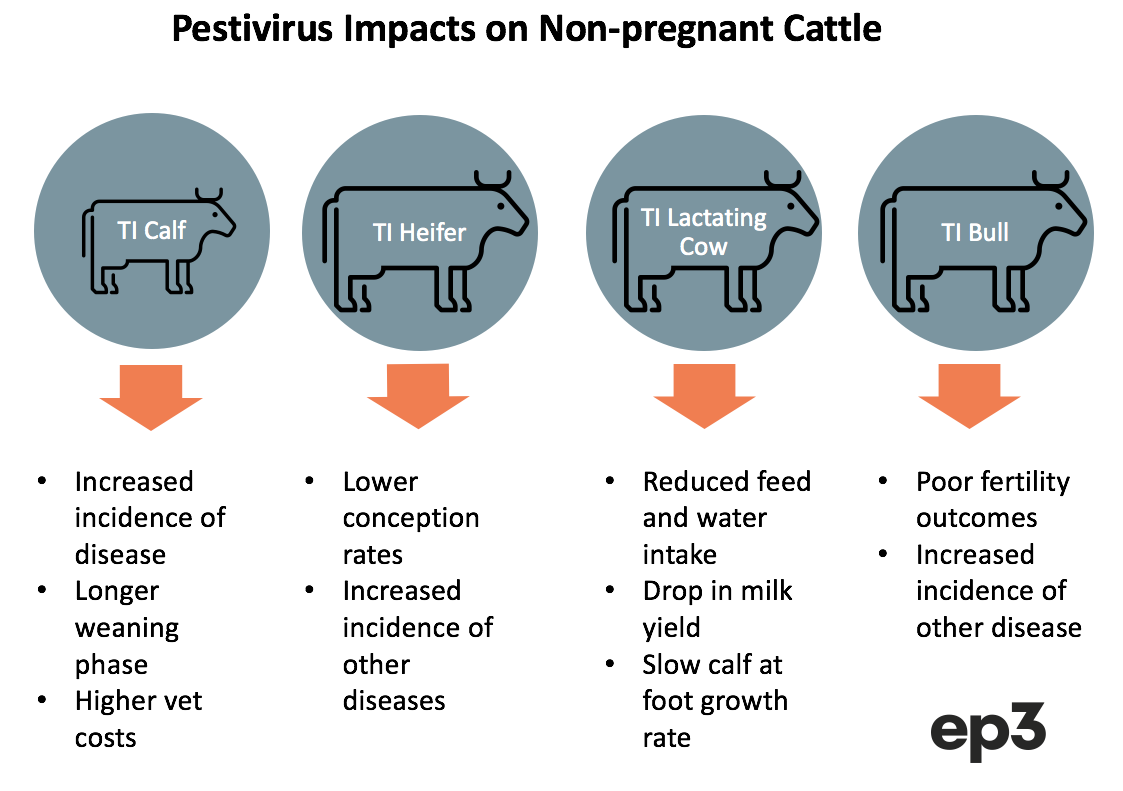
Impact of Pestivirus on non-pregnant cattle
Susceptible cattle that are exposed to Pestivirus and become transiently infected (TI) can face a variety of negative impacts, dependent upon the type of cattle they are, before they recover from the virus and become immune.
TI calves that are under a year old can have their immune system supressed making them more exposed to health issues like scouring, pneumonia, pinkeye, and poor growth rates. This can add to treatment costs and take these calves longer to reach their weaning phase.
Heifers that become TI during the mating phase can yield lower conception rates due to their exposure to Pestivirus. Meanwhile, lactating cows that become TI often reduce their feed and water intake until they have recovered from the virus (usually 2-3 weeks). This can cause a drop in milk yield for the TI period and slow the growth rate of any calves at foot.
Pestivirus infection can cause reduced fertility in TI bulls as the health impact of the viral load encourages less mating activity and compromises semen quality. Bulls may also be more susceptible to other disease during their TI phase. TI bulls may also develop persistent testicular infections with pestivirus in a small percentage of bulls, resulting in them shedding pestivirus in their semen after they develop antibodies and clear the infection from their system.
Despite some of these negative impacts upon TI cattle it is often difficult to clearly identify the direct cost of Pestivirus on the herd as animals that are not within their pregnancy phase can display few obvious symptoms. Sometimes the impact of Pestivirus can be obscured by other factors that also result in poor growth rates, common calfhood diseases, low milk production and infertility issues.
Similarly, Pestivirus has been implicated in the increased susceptibility of the cattle herd to Bovine Respiratory Disease (BRD). Two large Australian feedlot studies have demonstrated increased risk of BRD in cattle with recent exposure to pestivirus.
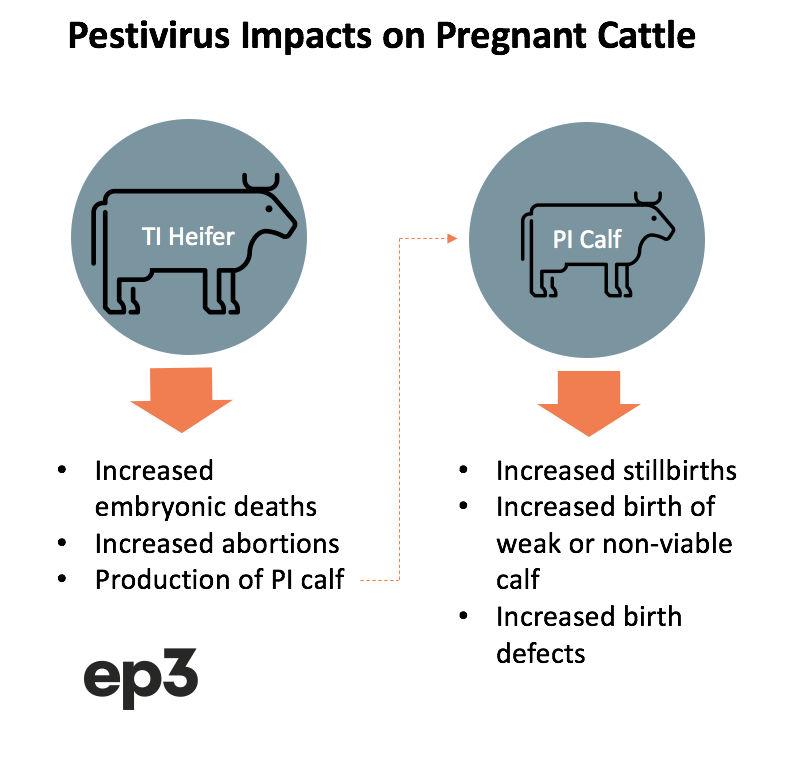
Impact of Pestivirus on pregnant cattle
Significant complications arise when Pestivirus infection impacts a cow during the pregnancy as the virus infects the calf foetus. Depending upon the time frame of the exposure there can be a variety of results from the exposure to the unborn calf.
In the early stage of pregnancy (0 to 40 days gestation) the Pestivirus infection can encourage embryonic death. During the middle of the pregnancy (40-120 days gestation) the foetus can face the increased likelihood of abortion.
However, if the foetus survives it can often be left with an immune system that does not create antibodies to fight the virus. In this case the calf can be born as persistently infected (PI) cattle that continues to shed the virus and remains infectious to any susceptible cattle in their proximity for the rest of their life.
Exposure to Pestivirus during late pregnancy (120-280 days gestation) can result in an increased number of stillborn calves, a higher incidence of birth defects and a higher number of calves that are born weak, with poor growth outcomes, or that are non-viable and die at an early age.
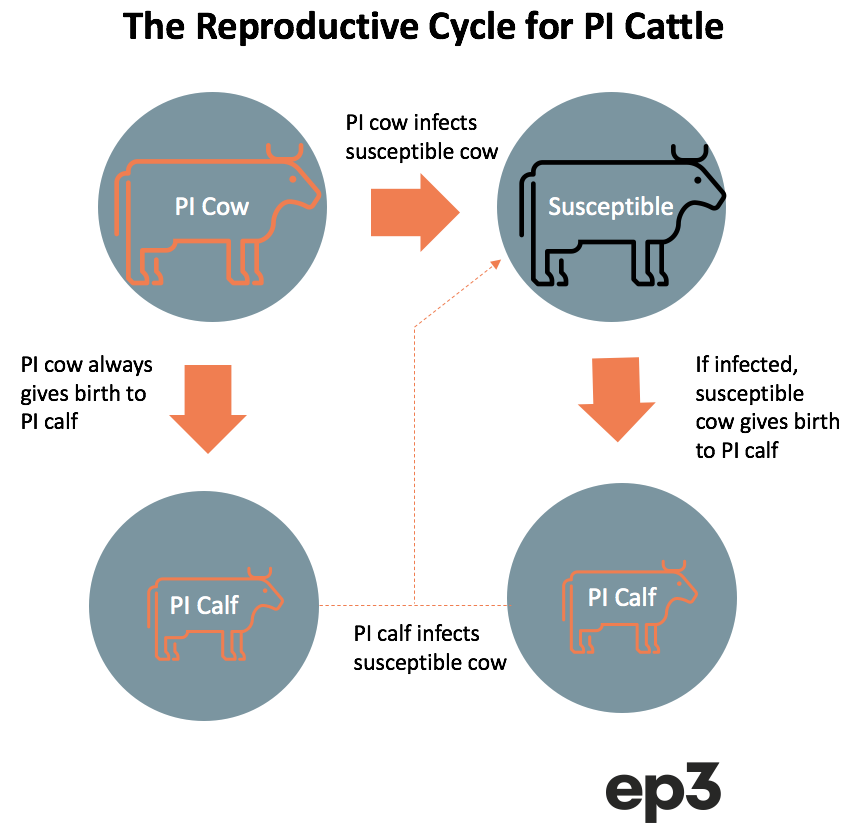
Persistently Infected versus Transiently Infected
Persistently infected (PI) cattle are the primary reservoir for Pestivirus, and they are the main reason that susceptible cattle become exposed to the virus, as transiently infected (TI) cattle are only infectious to other cattle for a handful of days and produce a fraction of the quantity of virus that PI cattle do. Without the existence of PI cattle, the spread of Pestivirus would be much easier to contain/manage and perhaps even eradicate.
A herd with PI cattle can lead to Pestivirus exposure to any susceptible heifers/cows during the reproductive phase, leading to the creation of further PI calves in-utero. While the heifer/cow will recover and remain immune to Pestivirus the calf can be born as a PI animal, constantly shedding the virus, and infecting other susceptible cattle.
Most PI cattle will either die from the development of mucosal disease early in their life or be culled from the herd for poor performance. In fact, most persistently infected calves don’t live to 12 months old. However, some PI cattle can present relatively normally, so it can be difficult to identify these viral reservoirs without appropriate testing.
If a PI heifer survives long enough to fall pregnant and give birth, they will produce a PI calf. In a herd with an active Pestivirus spread it is estimated that only 5% of breeding cattle will become infected during their reproductive phase, but this is enough to keep the virus circulating amongst the herd with any PI progeny continuing to infect any susceptible cattle that come within their proximity.
Breaking this cycle of infection between PI calves or older cattle and susceptible breeding age female cattle is critical to controlling the disease.
Control or Chaos?
Despite the prevalence of Pestivirus in the Australian herd, the impact it has upon cattle, and the cost to the sector there is no standard industry wide disease control messaging. There are questions on the national cattle health declaration relating to testing to identify PI animals, antibody levels and vaccination status. However, there are no standard definitions of the level of infection, or risk of infection within the herd, which makes it difficult for producers to assess the risk of disease on health declaration forms or when trading cattle.
The lack of a clear and concise national control strategy and uniform language for dealing with Pestivirus means that cattle producers are confronted with a myriad of options, with many opting to do nothing. The logic behind this strategy appears to favour the view that once their herd is infected with Pestivirus the disease takes care of itself in terms of creating immunity across the exposed TI cattle. However, numerous studies have shown that levels of exposure in breeding herds vary and very few are able to maintain elevated levels of exposure across all breeding females for an extended period, resulting in ongoing losses.
Levels of exposure to pestivirus appear to have changed little in the Australian cattle herd since they were first reported in 1968, despite attempts to raise awareness of the disease amongst producers for the past two decades. Knowledge of the disease coupled with the availability of effective, accurate and inexpensive tests and an effective vaccine make the disease very controllable.
Alternative strategies can involve a combination of testing for immunity or PI animals, removing PI animals from the herd and undertaking targeted vaccination programs – either for the entire herd, for susceptible cattle within the herd or for susceptible cattle during the breeding phase.
Although elements of the lifecycle of Pestivirus may be complex, management of the disease using a monitoring, eliminating and vaccination program is an effective, easily accessible, and simple methodology to deal with the virus. A program of this nature has benefits over and above cost/loss minimisation in terms of increased animal welfare standards and maintenance of social licence.
The EP3 team have scheduled additional articles that will investigate the impact of Pestivirus on the beef and dairy sector. This will include estimates of the economic cost on the Australian cattle industry and a summary of control/eradication programs being undertaken overseas to effectively manage the virus.